News Articles - GRADE
At home “Capillary kits” developed and validated for HbA1c testing in GRADE
The GRADE and EDIC Study researchers designed and completed a study to compare HbA1c values obtained from capillary blood collection kits versus venous whole blood collections in study participants with type 1 or type 2 diabetes.
Mean glycemia (mean blood glucose) is a key factor in the development of long-term complications in type 1 and type 2 diabetes. Glycated hemoglobin (HbA1c) is the most widely used clinical test to estimate mean blood glucose. It is measured from a blood sample and reflects the average blood sugar level over the past two to three months. HbA1c is used in clinical care to manage and study diabetes. As part of an effort to ensure more complete data collection and HbA1c measurement for participants who are unable to attend all study visits in person, the GRADE researchers developed and evaluated an at-home blood sample collection kit. The kit would allow participants to collect capillary blood samples by fingerstick and mail them to a central laboratory, where HbA1c would be measured using the same laboratory methods used on the venous whole blood samples collected in the clinic by the study staff.
The purpose of the study was to test the reliability of the kit and see if it could be used in place of an in-clinic blood sample draw for valid, accurate results. The study found that the capillary blood HbA1c result compared well with the results from the venous whole blood collected in clinic. Most participants felt the kits were easy to use. Following this validation study, the capillary kit was used widely in both the GRADE and EDIC studies when COVID-19 related restrictions did not allow for in-person study visits.
Publication Citation
Nathan DM, Krause-Steinrauf H, Braffett BH, Arends VL, Younes N, McGee P, Lund C, Johnson M, Lorenzi G, Gao X, Steffes MW, Lachin JM, GRADE Research Group, DCCT/EDIC Research Group. Comparison of central laboratory HbA1c measurements obtained from a capillary collection versus a standard venous whole blood collection in the GRADE and EDIC studies. PloS One, 2021;16(11) PMCID:PMC8592405
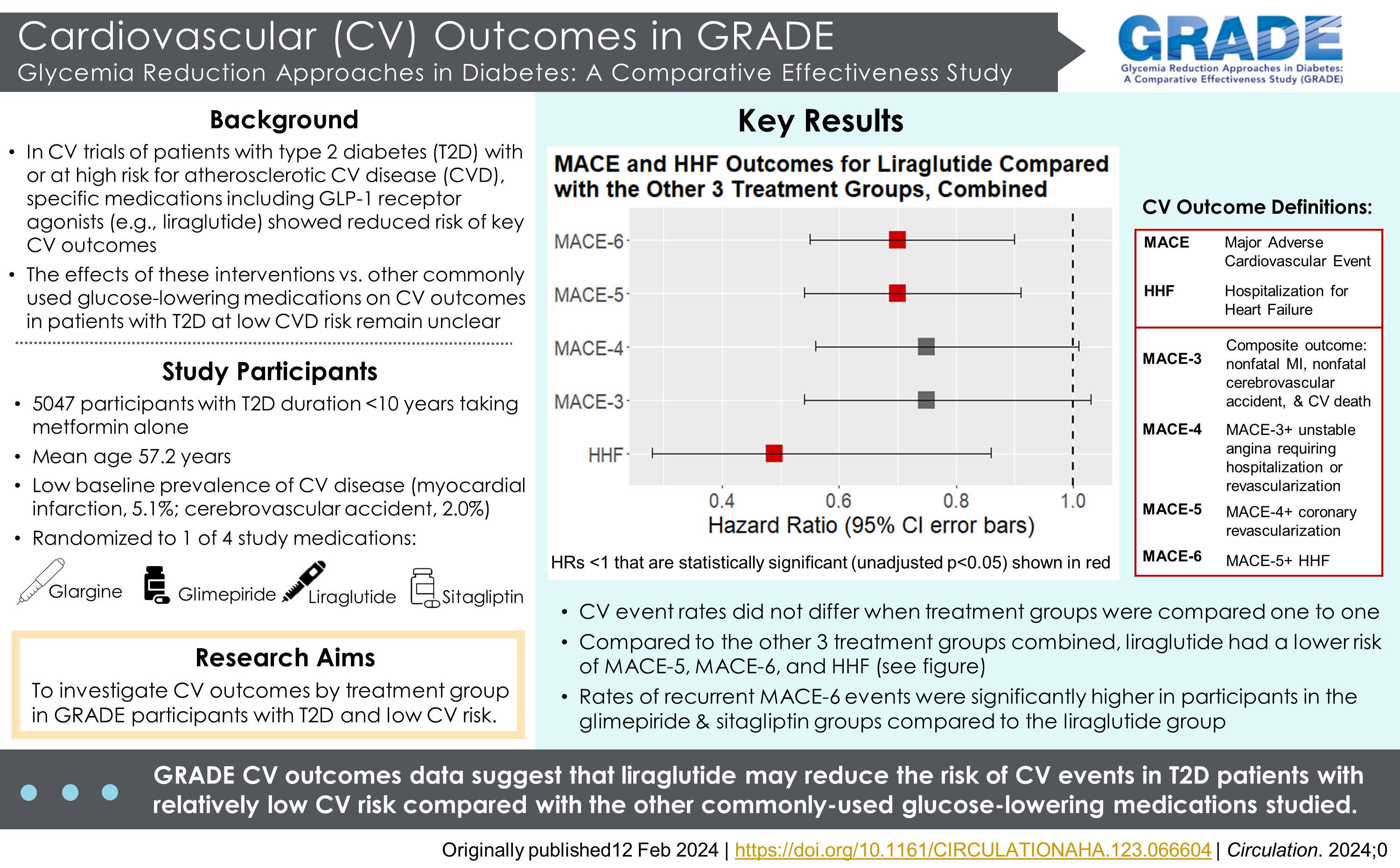
Publication Citation
Green JB, Everett BM, Ghosh A, Younes N, Krause-Steinrauf H, Barzilay J, Desouza C, Inzucchi SE, Pokharel Y, Schade D, Scrymgeour A, Tan MH, Utzschneider KM, Mudaliar S, and the GRADE Study Research Group. Cardiovascular Outcomes in GRADE (Glycemia Reduction Approaches in Type 2 Diabetes: A Comparative Effectiveness Study). Circulation 2024: Feb 12. Doi: 10.1161/CIRCULATIONAHA.123.066604. PMID: 38344820.

Publication Citation
Pop-Busui R, Rosin SP, Butera NM, Krause-Steinrauf H, Abou Assi H, Garg RK, Inzucchi SE, Katona A, McGill JB, Mudaliar S, Schade DS, Seaquist ER, Tiktin M, Soliman EZ, Green JB, GRADE Research Group. Differences in prevalence and incidence of rlectrocardiogram abnormalities and cardiovascular autonomic neuropathy among randomized glucose-lowering treatments in early type 2 diabetes: The Glycemia Reduction Approaches in Diabetes (GRADE) cohort. Diabetes Care. 2025 Nov 1; 48(11):1960-1970. Doi: 10.2337/dc25-1087 . PMID: 40986645.
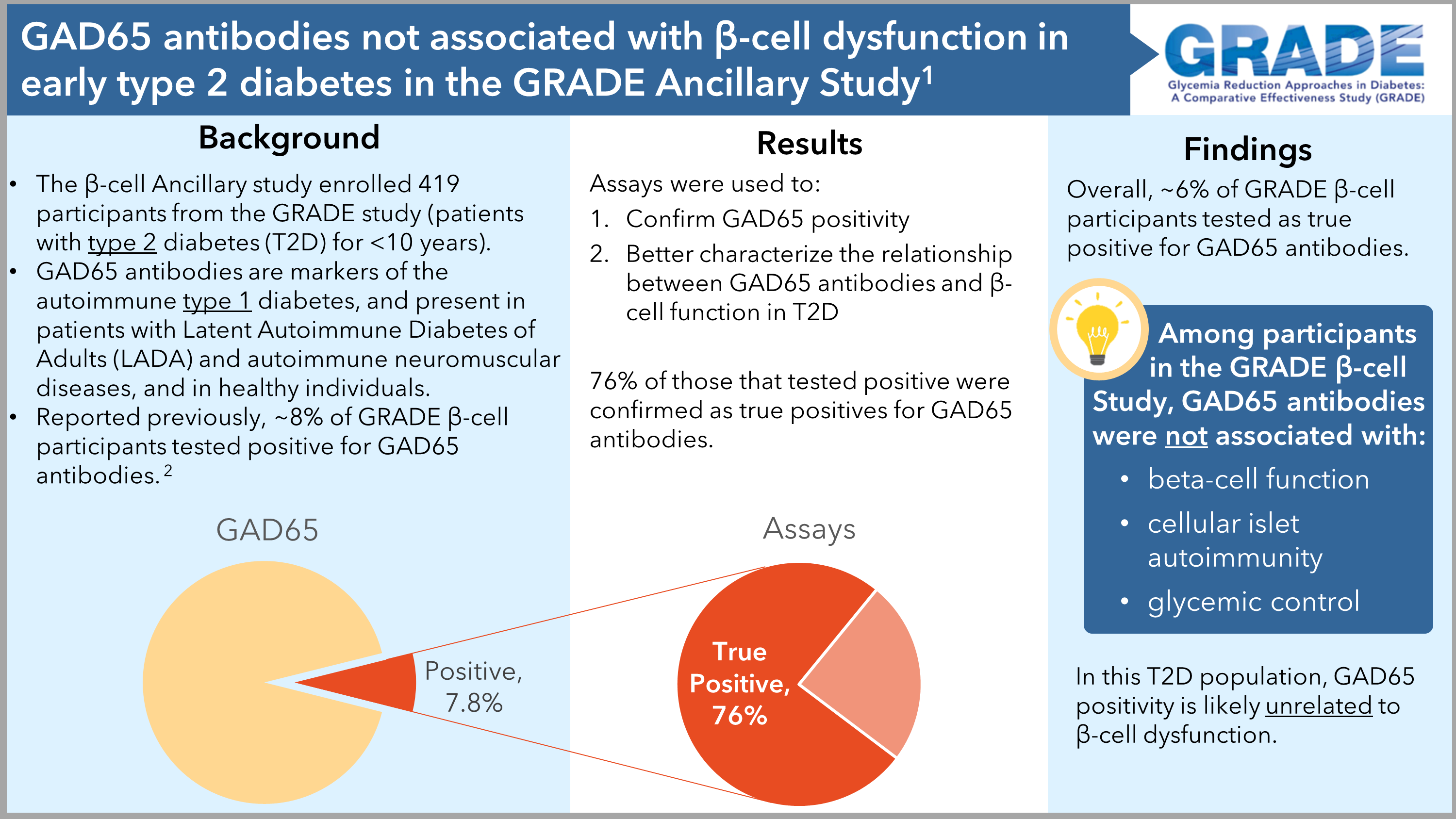
Publication Citation
1. Hampe CS, Shojaie A, Brooks-Worrell B, Dibay S, Utzschneider KM, Kahn SE, Larkin ME, Johnson ML, Younes N, Rasouli N, DeSouza C, Cohen RM, Park JY, Florez HJ, Valencia WM, Stempel R, Palmer JP, Balasubramanyam A, and the GRADE Research Group. GAD65Abs Are Not Associated With Beta-Cell Dysfunction in Patients With T2D in the GRADE Study. J. Endocr. Soc. 2024 Feb 8: 8(3).Doi: 10.1210/jendso/bvad179. PMID: 38333889. PMCID: PMC10853002.
2. Brooks-Worrell B, Hampe CS, Hattery EG, Palomino B, Zangeneh SZ, Utzschneider KM, Kahn SE, Larkin ME, Johnson ML, Mather KJ, Younes N, Rasouli N, Desouza C, Cohen RM, Park JY, Florez HJ, Valencia WM, Shojaie A, Palmer JP, Balasubramanyam A, and the GRADE Beta-Cell Ancillary Study Network. Islet Autoimmunity is Highly Prevalent and Associated With Diminished β-Cell Function in Patients With Type 2 Diabetes in the GRADE Study. Diabetes (New York, NY). 71(6):1261-1271. PMID: 35061024. PMCID:PMC9375448.

Publication Citation
Hsia DS, Younes N, Ghosh A, Kazemi EJ, Krause-Steinrauf H, Buse JB, Baker C, Brown-Friday J, Diaz E, Diner J, Groessl J, Legowski EA, Mariash CN, Waltje AH, Wexler DJ, Martin CL, GRADE Research Group. Association of Hospitalizations With Randomized Glycemia-Lowering Treatment in GRADE. Diabetes Care. 2025 Jul 1; 48(7):1288-1294. Doi: 10.2337/dc24-2839 . PMID: 40424051.
Islet Autoimmunity Is Highly Prevalent and Associated With Diminished b-Cell Function in Patients With Type 2 Diabetes in the GRADE Study

Deficiencies in insulin secretion by beta cells of the islets of Langerhans (clusters of cells found in the pancreas) is critical to the development of both type 1 and type 2 diabetes (T2D). Immune system-generated attacks directed against tags on the surface of these clusters of beta cells (“antigens”) is thought to be the underlying root cause behind insufficient insulin secretion in type 1 diabetes—which is why type 1 diabetes is considered to be an “autoimmune disease”. While autoimmunity against beta cells has not traditionally been considered a significant underlying defect in type 2 diabetes, the GRADE Beta Cell ancillary study was done to see to what degree islet autoimmunity might play a role in beta cell dysfunction in type 2 diabetes. The researchers looked at the relationship between beta cell function measurements from blood samples collected from a timed oral glucose tolerance test (OGTT) and humoral and cellular islet immunity measured from blood samples. They found that cellular islet autoimmunity is much greater in patients with T2D than previously reported. In addition, they found that T-cell mediated autoimmunity is associated with lower beta cell function and worse blood sugar (glycemic) control. These findings may reveal an immune-based mechanism that could contribute to beta cell dysfunction in type 2 diabetes, define different subtypes of type 2 diabetes, and uncover new approaches to treat this complex condition.
Publication Citation
Brooks-Worrell B, Hampe CS, Hattery EG, Palomino B, Zangeneh SZ, Utzschneider KM, Kahn SE, Larkin ME, Johnson ML, Mather KJ, Younes N, Rasouli N, Desouza C, Cohen RM, Park JY, Florez HJ, Valencia WM, Shojaie A, Palmer JP, Balasubramanyam A, and the GRADE Beta-Cell Ancillary Study Network. Islet Autoimmunity is Highly Prevalent and Associated With Diminished β-Cell Function in Patients With Type 2 Diabetes in the Grade Study. Diabetes (New York, NY ), 2022;PMCID:PMC9375448
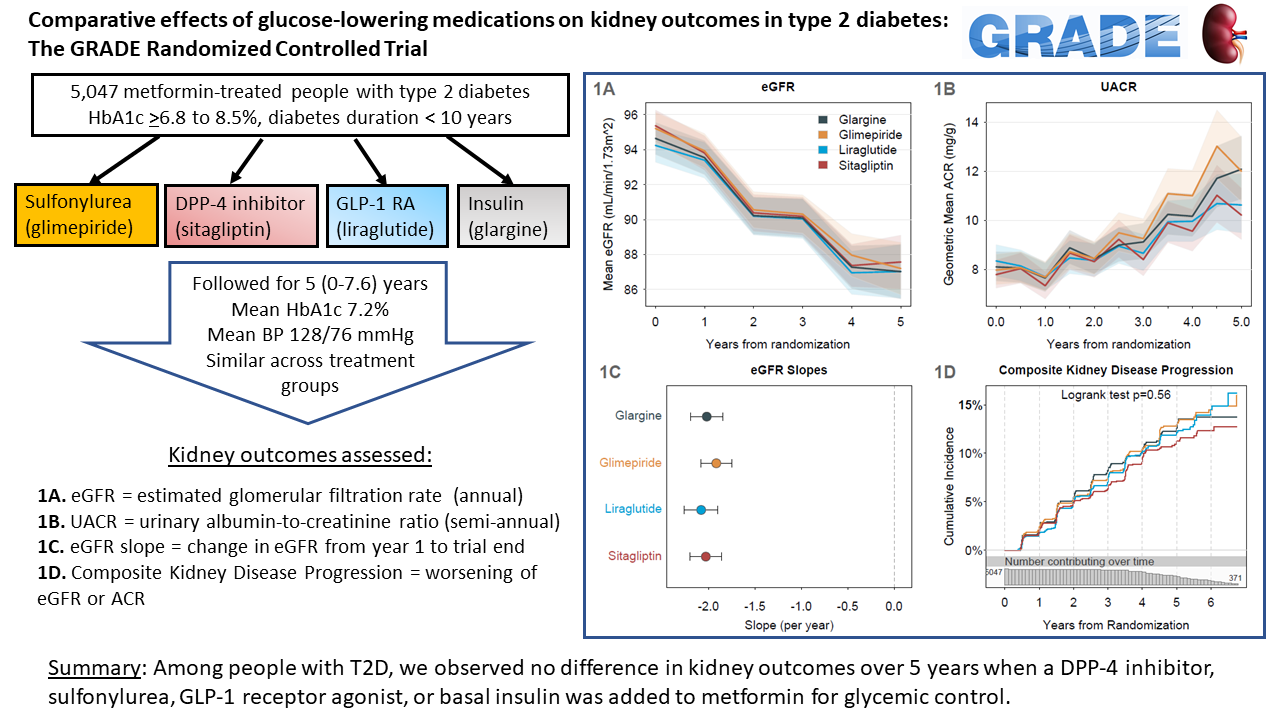
Publication Citation
Wexler DJ, deBoer IH, Ghosh A, Younes N, Bebu I, Inzucchi SE, McGill JB, Mudaliar S, Schade D, Steffes MW, Tamborlane WV, Tan MH, Ismail-Beigi F, for the GRADE Research Group. Comparative Effects of Glucose-Lowering Medications on Kidney Outcomes in Type 2 Diabetes. JAMA IM, May 22 2023; PMCID:PMC37213109
Publication Citation
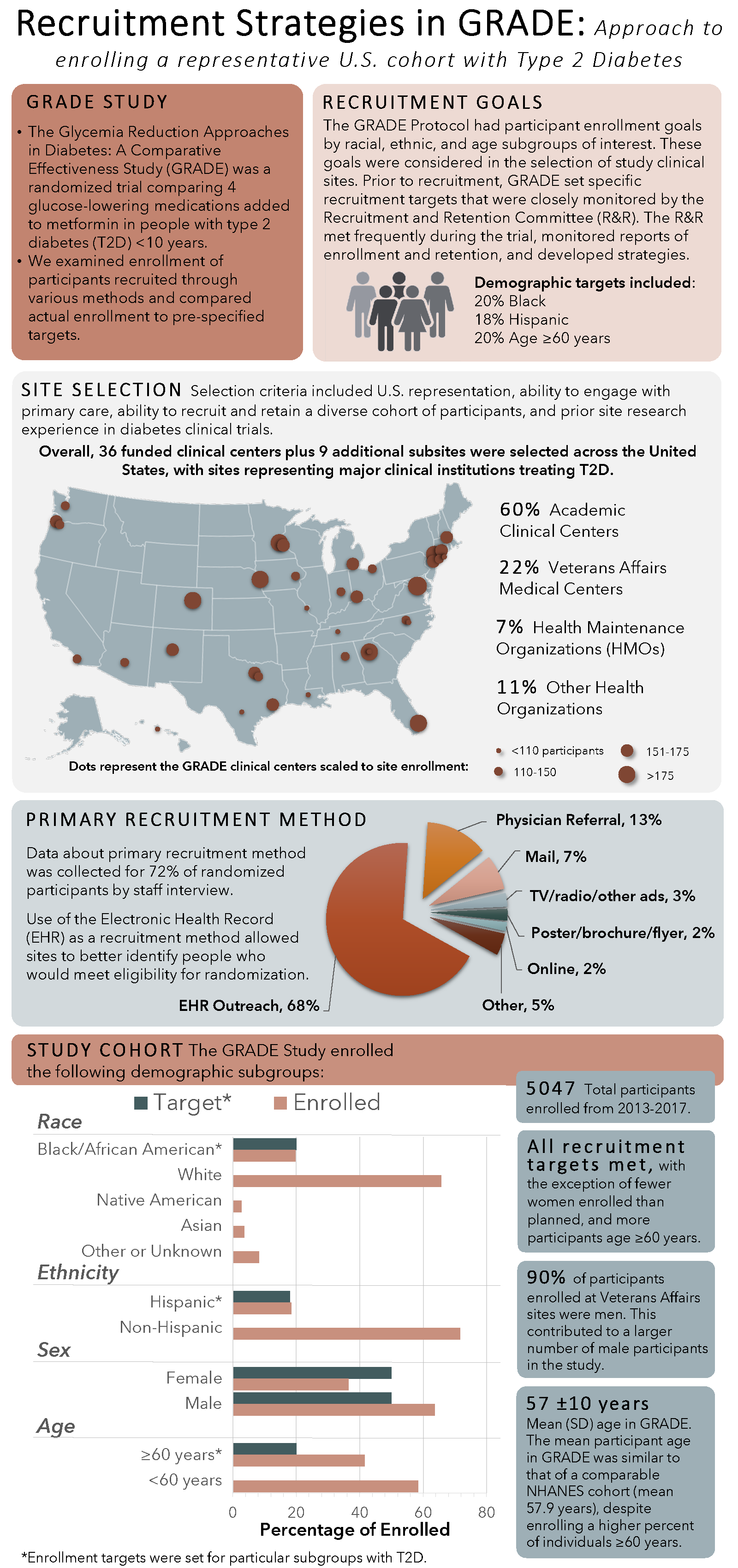
1. Cherrington AL, Krause-Steinrauf H, Aroda V, Buse JB, Fattaleh B, Fortmann SP, Hall S, Hox SH, Kuhn S, Killean T, Loveland A, Phillips LS, Uy Jackson A, Waltje A, McKee MD and the GRADE Research Group. Use of comprehensive recruitment strategies in glycemia reduction approaches in diabetes: A comparative effectiveness study (GRADE) multi-center clinical trial. Clinical Trials. 2023 Jun 17; doi: 10.1177/17407745231175919. PMID: 37329282; PMCID: PMC10524662.
2. Wexler DJ, Krause-Steinrauf H, Crandall JP, Florez HJ, Hox SH, Kuhn A, Sood A, Underkofler C, Aroda VR, the GRADE Research Group. Baseline Characteristics of Randomized Participants in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care. 2019;11(43):2098-2107. PMID 31391203; PMCID:PMC6804613
Publication Citation
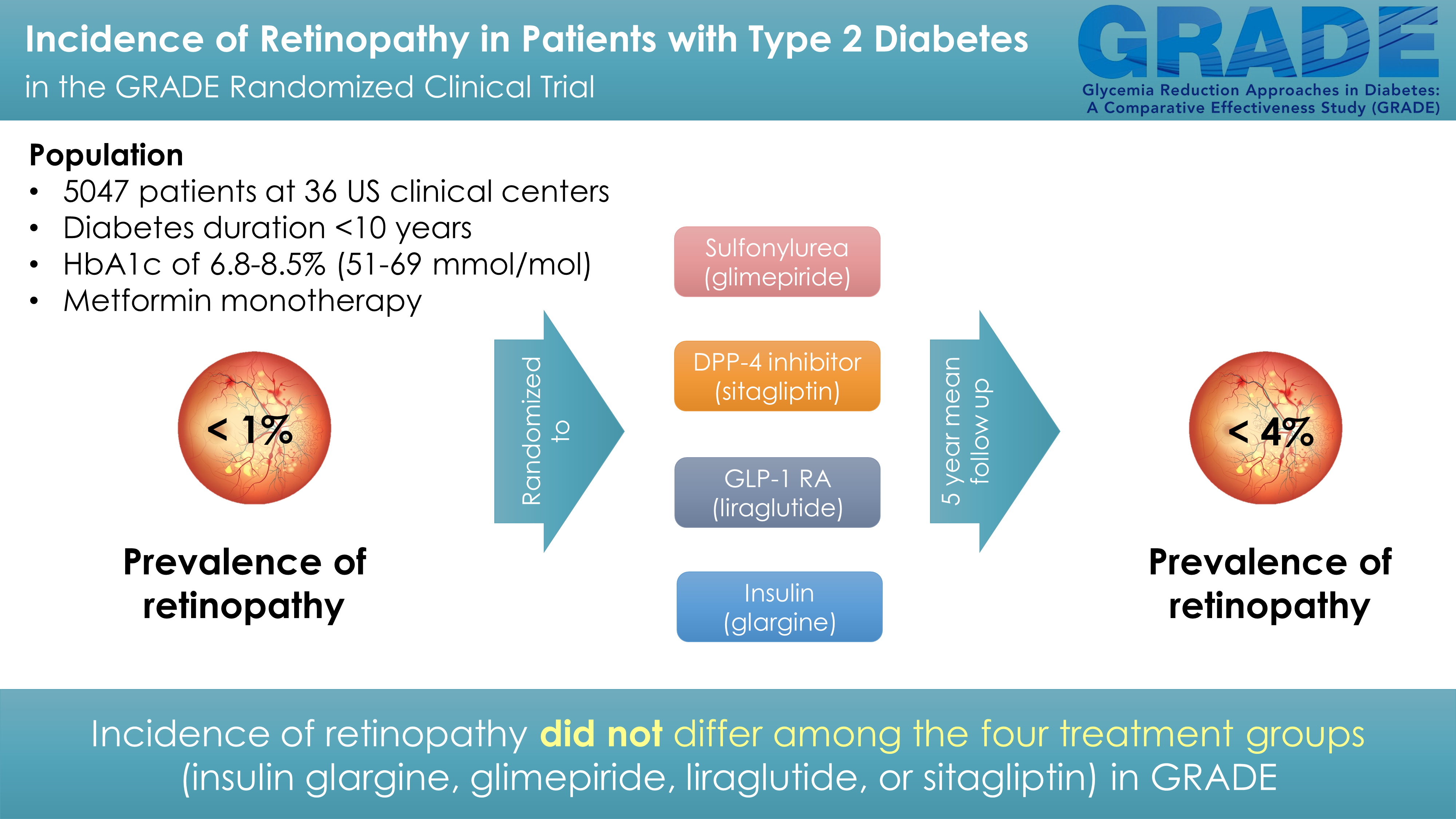
Hsia DS, Younes N, Krause-Steinrauf H, Sayyed Kassem L, for the GRADE Research Group. The Incidence of Retinopathy in the Glycemia Reduction Approaches in Type 2 Diabetes (GRADE) Study: A Randomized Comparative Effectiveness Study. J Diabetes Complications. 2024 Feb 5; 38 (3):108692. Doi: 10.1016/j.jdiacomp.2024.108692. Epub ahead of print. PMID: 38354481.

Publication Citation
Wexler DJ, Garvey WT, Ghosh A, Kazemi EJ, Krause-Steinrauf H, Ahmann AJ, Brown-Friday J, Casula S, Cherrington AL, Elasy TA, Fortmann SP, Krakoff JA, Mudaliar S, Tiktin M, Younes N, GRADE Study Research Group. Weight Gain Was Associated With Worsening Glycemia and Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes Independent of Diabetes Medication in the GRADE Randomized Controlled Trial. Diabetes Care. 2025 Apr 23;48(6):935–944. Doi: 10.2337/dc24-2825 . PMID: 40267365.
Publication Citation
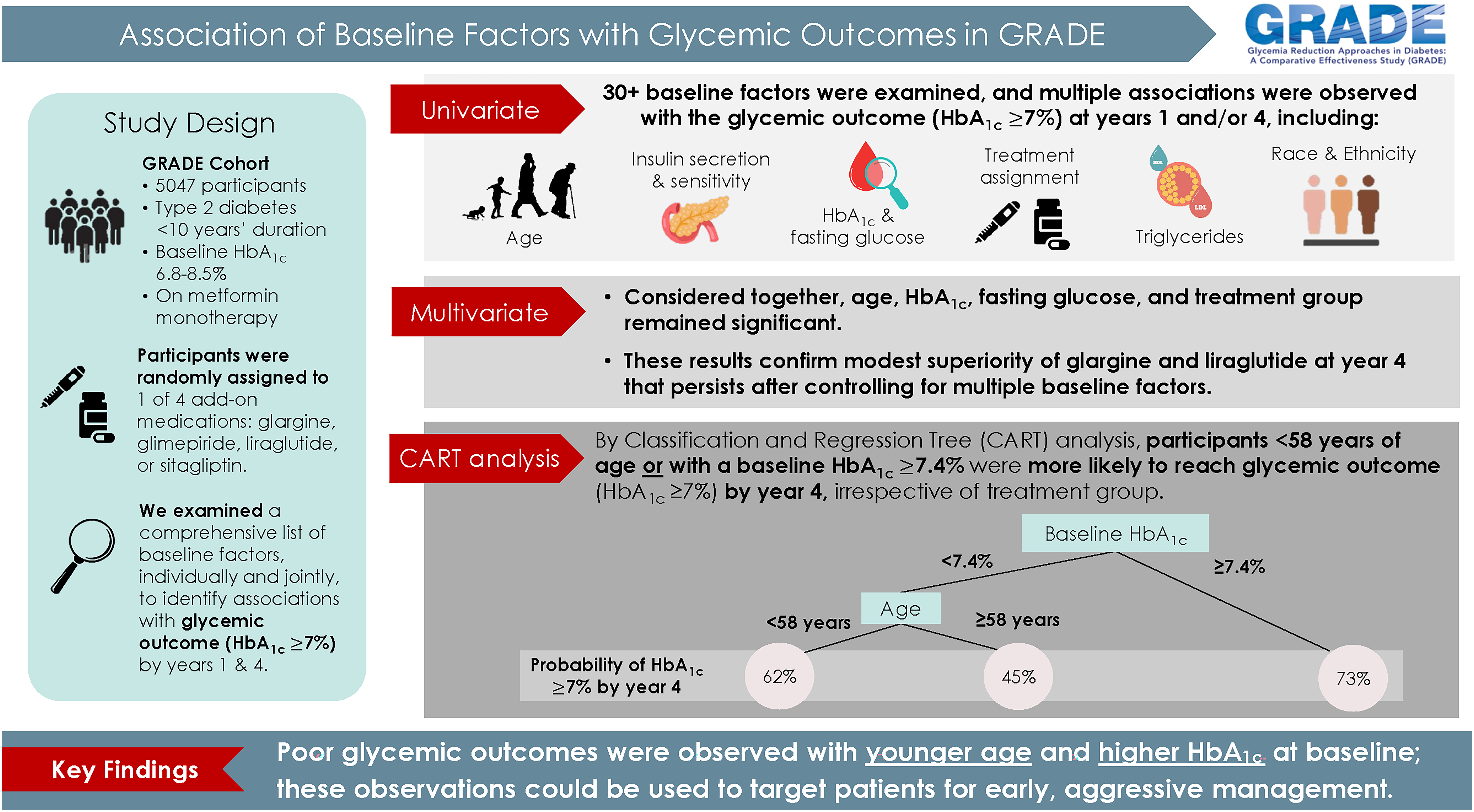
Garvey WT, Cohen RM, Butera NM, Kazemi EJ, Younes N, Rosin SP, Suratt CE, Ahmann A, Hollander PA, Krakoff J, Martin CL, Seaquist E, Steffes MW, Lachin JM, for the GRADE Research Group. The Association of Baseline Factors with Glycemic Outcomes in the GRADE Study: A Comparative Effectiveness Randomized Clinical Trial. Diabetes Care 2024 Apr 1; 47(4)562-570. Doi: 10.2337/dc23-1782. Epub ahead of print. PMID: 38285957.
Publication Citation
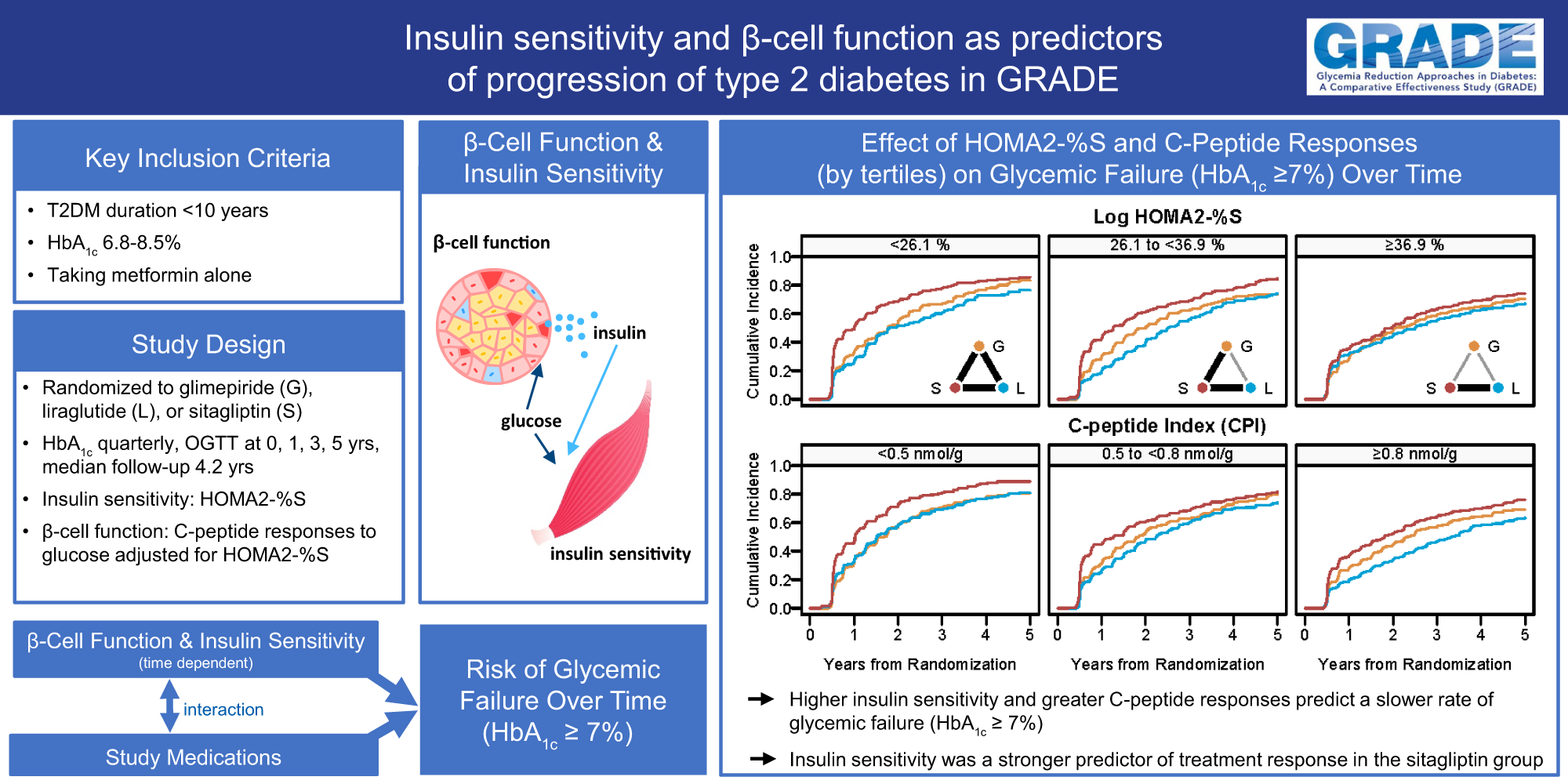
Utzschneider KM, Younes N, Butera NM, Balasubramanyam A, Bergenstal RM, Barzilay J, DeSouza C, DeFronzo RA, Elasy T, Krakoff J, Kahn SE, Rasouli N, Valencia WM, Sivitz WI, for the GRADE Research Group. Impact of Insulin Sensitivity and β-Cell Function Over Time on Glycemic Outcomes in GRADE (Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study): Differential Treatment Effects of Dual Therapy. Diabetes Care. 2024 Apr 1; 47(4)571-579. doi: 10.2337/dc23-1059. Epub ahead of print. PMID: 38190619.
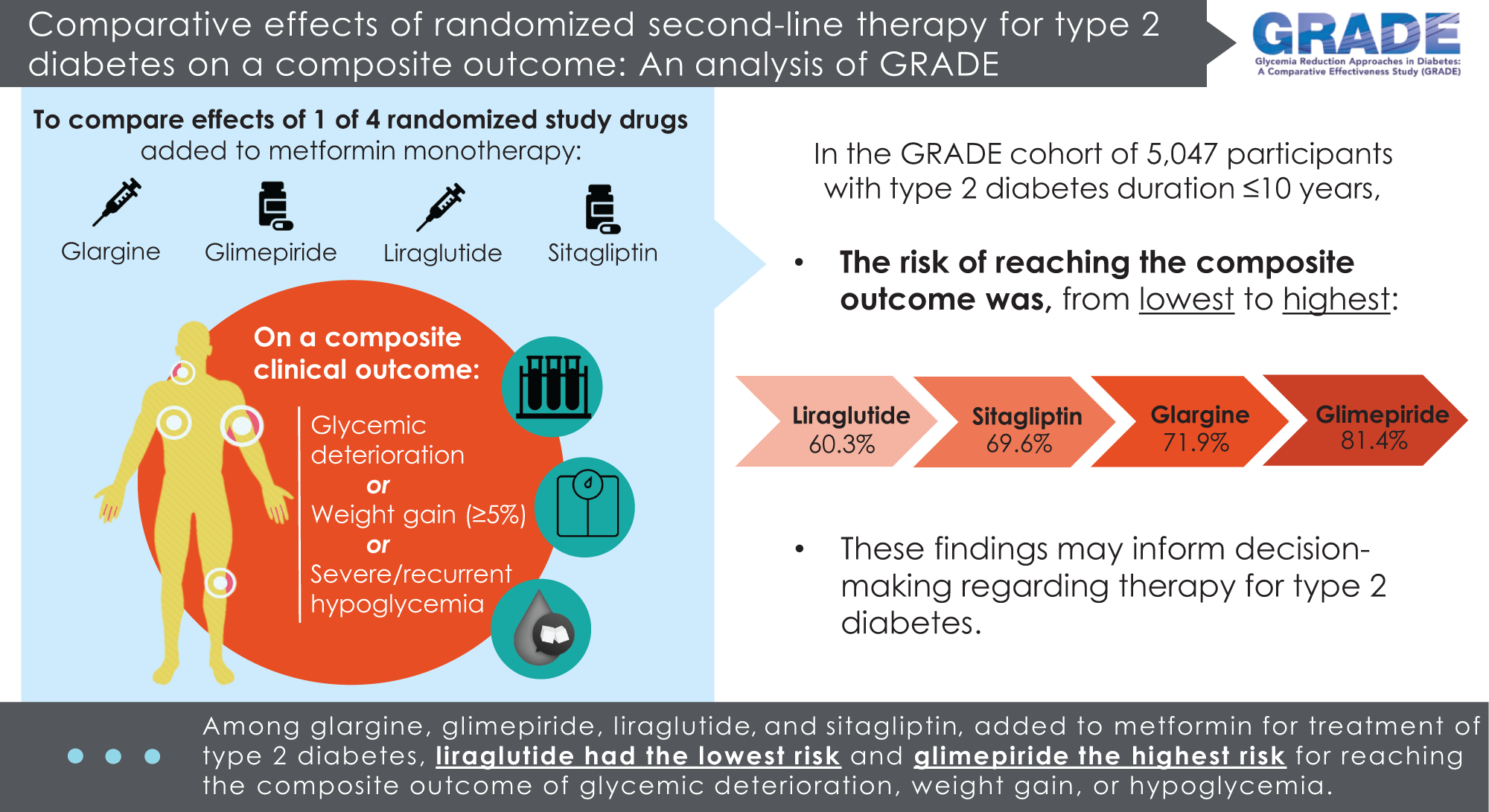
Publication Citation
Kirkman, MS, Tripputi M, Krause-Steinrauf H, Bebu I, AbouAssi H, Burch H, Duran-Valdez E, Florez HJ, Garvey WT, Hsia DS, Salam M, Pop-Busui R, for the GRADE Research Group. Comparative Effects of Randomized Second-line Therapy for Type 2 Diabetes on a Composite Outcome Incorporating Glycemic Control, Body Weight, and Hypoglycemia: An Analysis of Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care. 2024 Apr 1; 47(4)594-602. doi: 10.2337/dc23-1332. Epub ahead of print. PMID: 38194519.
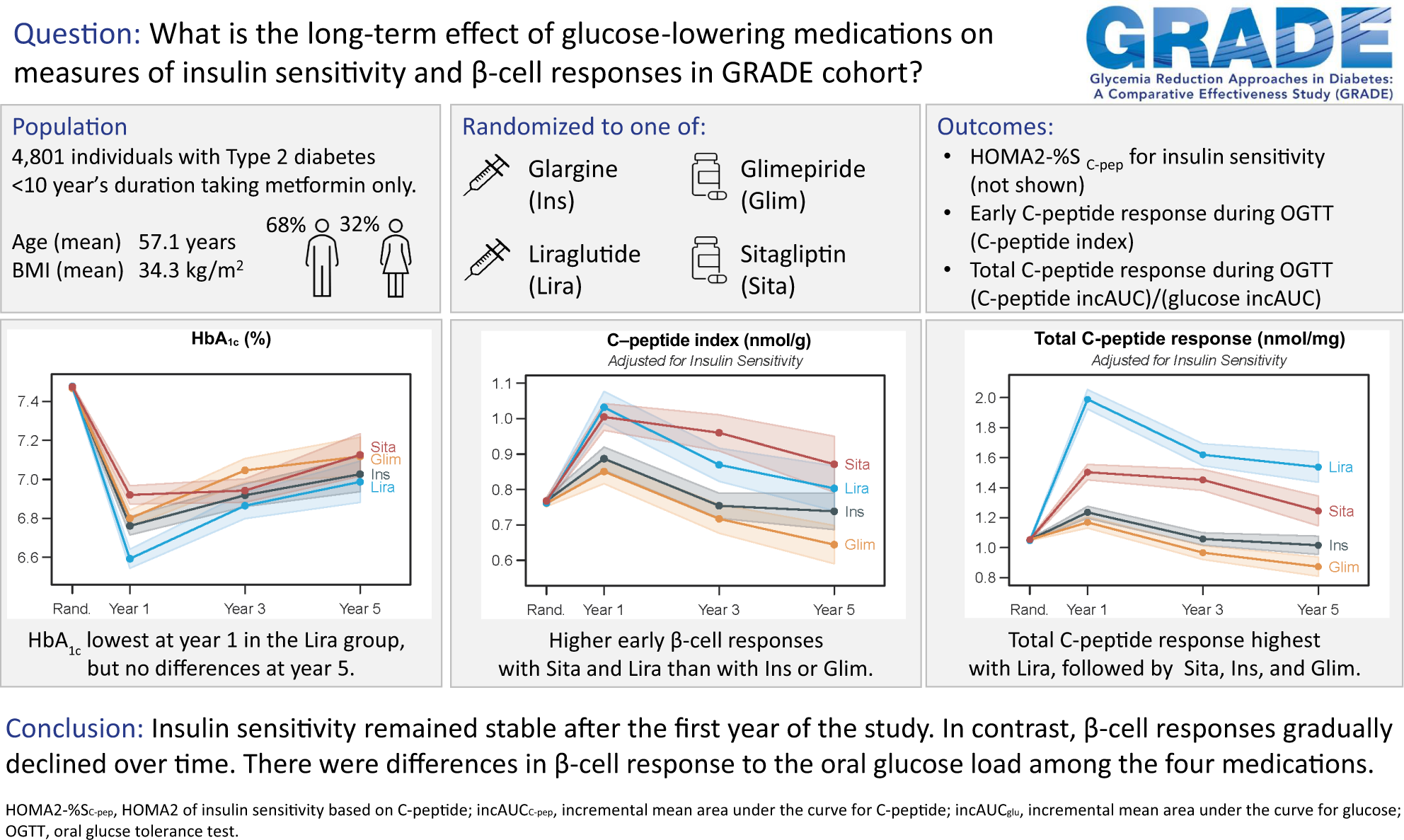
Publication Citation
Rasouli N, Younes N, Ghosh A, Albu J, Cohen RM, DeFronzo RA, Diaz E, Sayyed Kassem L, Luchsinger JA, McGill JB, Sivitz WI, Tamborlane WV, Utzschneider KM, Kahn SE, for the GRADE Research Group. Longitudinal Effects of Glucose-Lowering Medications on β-Cell Responses and Insulin Sensitivity in Type 2 Diabetes: The GRADE Randomized Clinical Trial. Diabetes Care. 2024 Apr 1; 47(4)580-588. doi: 10.2337/dc23-1070. Epub ahead of print. PMID: 38211595.
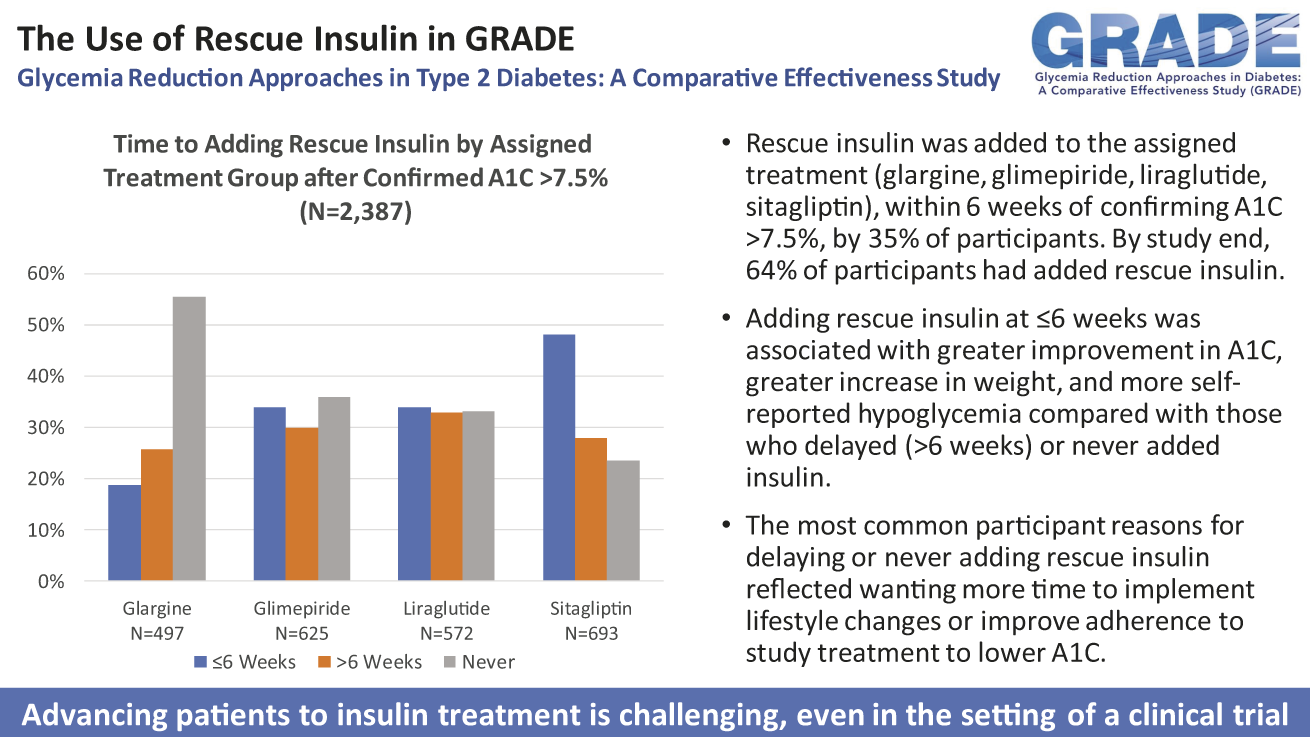
Publication Citation
Hollander PA, Krause-Steinrauf H, Butera NM, Kazemi EJ, Ahmann AJ, Fattaleh BN, Johnson ML, Killean T, Lagari VS, Larkin ME, Legowski EA, Rasouli N, Willis HJ, Martin CL; for the GRADE Research Group. The Use of Rescue Insulin in the Glycemia Reduction Approaches in Type 2 Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care. 2024 Apr 1; 47(4)638-645. doi: 10.2337/dc23-0516. Epub ahead of print. PMID: 37756542.
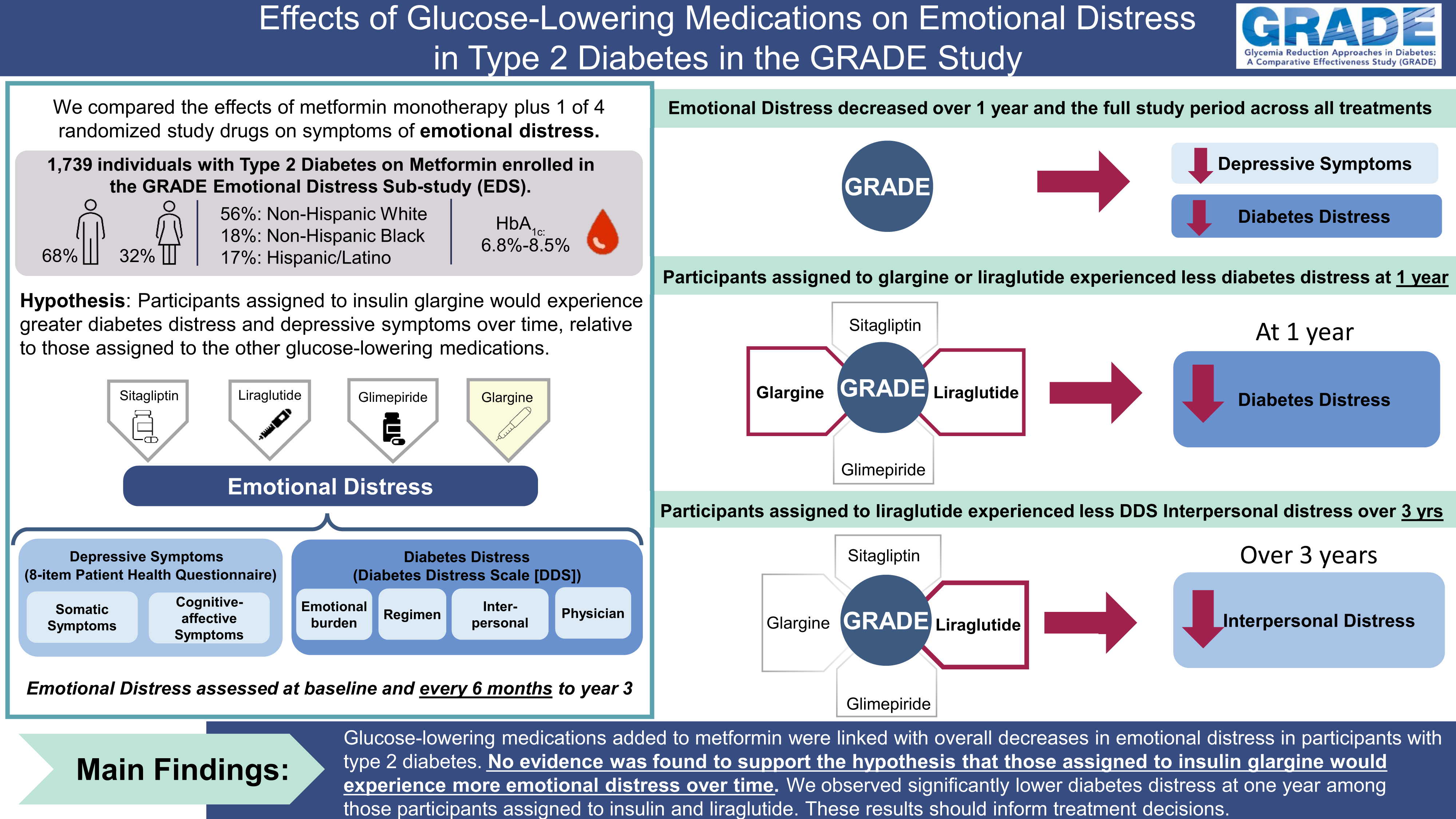
Publication Citation
Gonzalez JS, Bebu I, Krause-Steinrauf H, Hoogendoorn CJ, Crespo-Ramos G, Presley C, Naik AD, Kuo S Johnson MJ, Wexler D, Crandall JP, Bantle AE, Arends V, Cherrington A, for the GRADE Research Group. Differential Effects of Type 2 Diabetes Treatment Regimens on Diabetes Distress and Depressive Symptoms in GRADE: 2024 Apr 1; 47(4)610-619. Doi:/10.2337/dc23-2459. Epub ahead of print. PMID: 38416773.
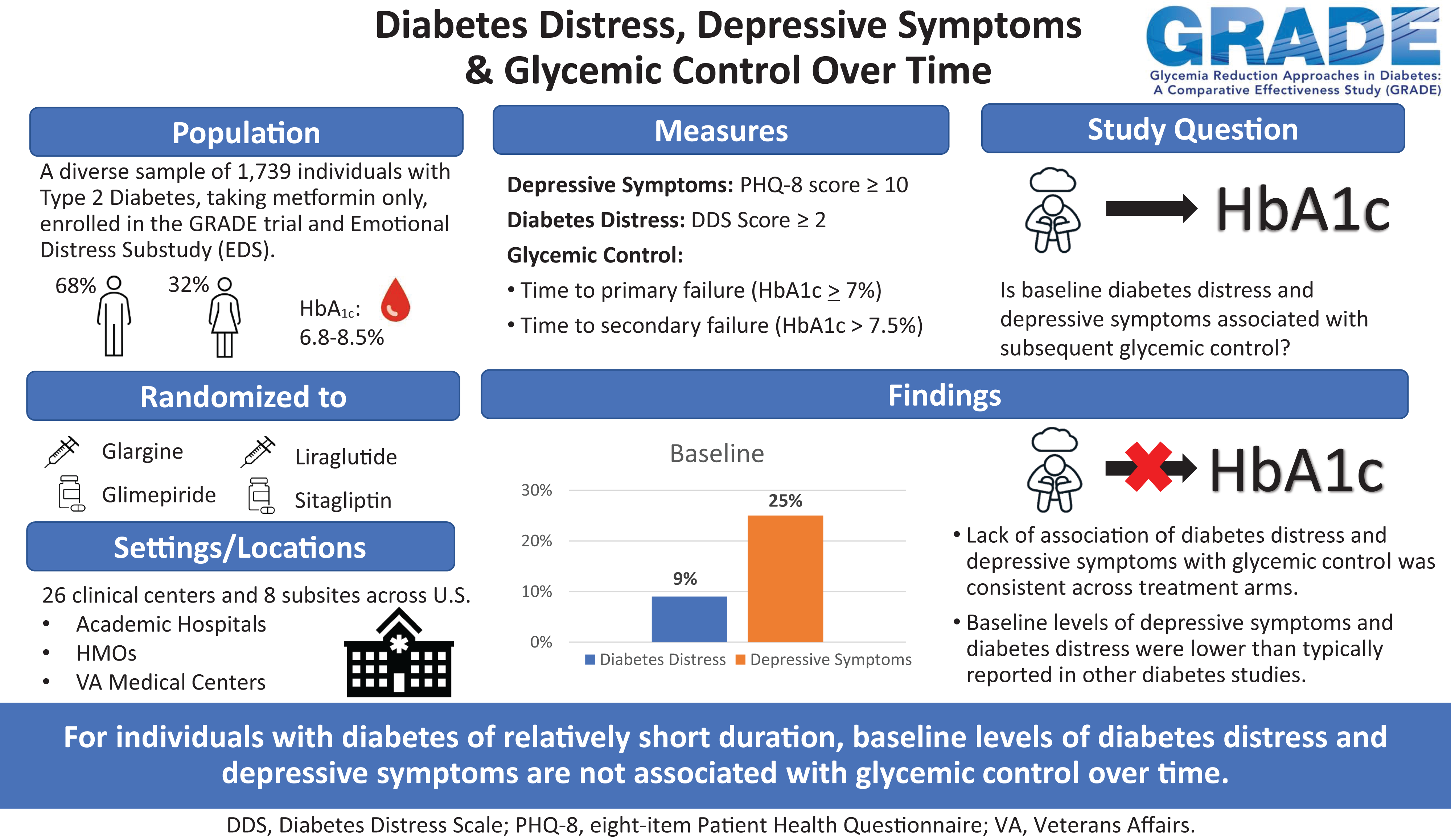
Publication Citation
Cherrington AL, Bebu I, Krause-Steinrauf H, Hoogendoorn CJ, Crespo-Ramos G, Presley C, Naik AD, Balasubramanyam A, Gramzinski MR, Killean T, Arends VL, Gonzalez JS, for the GRADE Research Group. Does emotional distress predict worse glycemic control over time? Results from the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study. Diabetes Care 2024 Apr 1; 47(4)620-628. Doi: 10.2337/dc23-0642 . Epub ahead of print. PMID: 38252848.
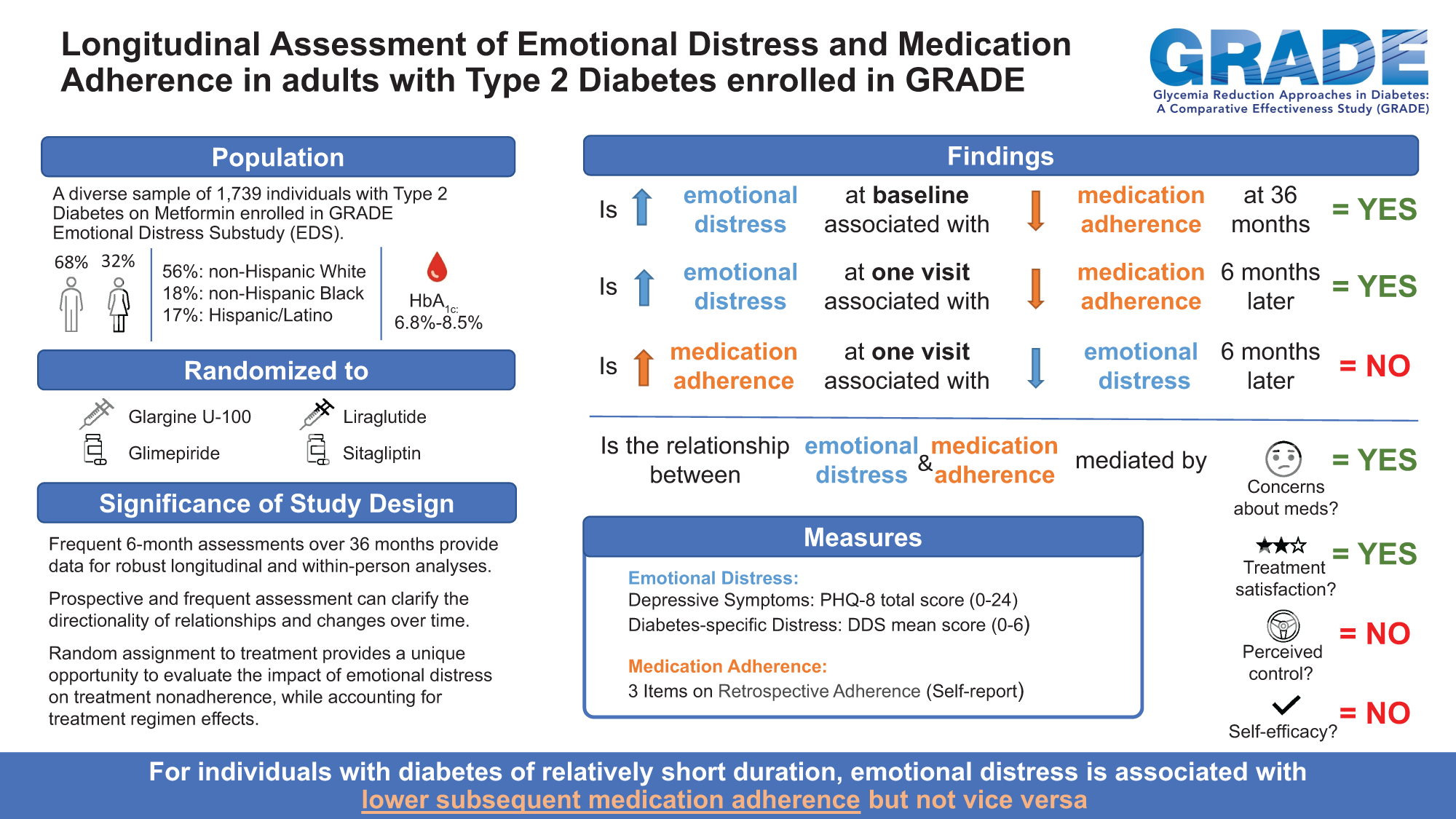
Publication Citation
Hoogendoorn CJ, Krause-Steinrauf, Uschner D, Wen H, Presley CA, Legowski EA, Naik AD, Hill Golden S, Arends VL, Brown-Friday J, Krakoff JA, Suratt CE, Waltje AH, Cherrington AL, Gonzalez JS, for the GRADE Research Group. Emotional Distress Predicts Reduced Type 2 Diabetes Treatment Adherence in Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care. 2024 Apr 1; 47(4)629-637. Doi: 10.2337/dc23-1401. Epub ahead of print. PMID: 38227900.
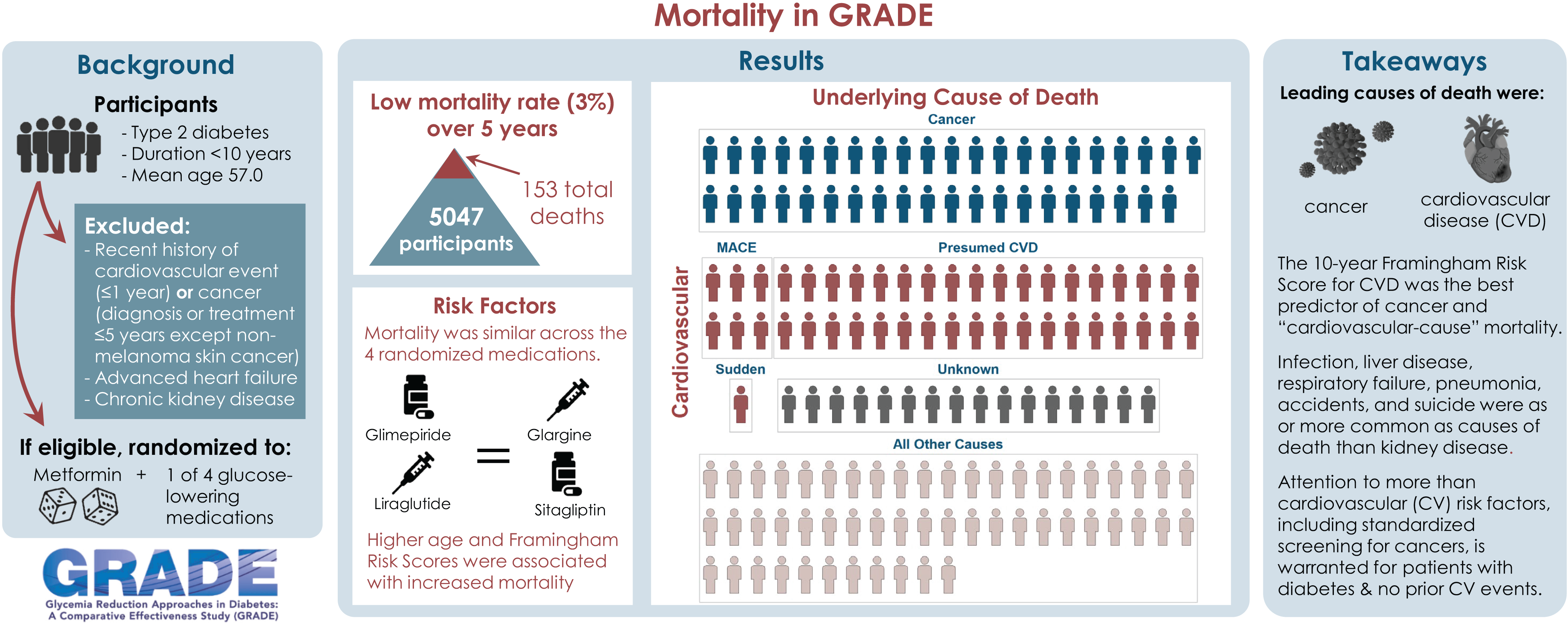
Publication Citation
Banerji MA, Buse JB, Younes N, Krause-Steinrauf H, Ghazi A, Lee M, Park J, Pop-Busui R, Underkofler C, Fortmann SP, for the GRADE Research Group. Mortality in the Glycemia Reduction Approaches in Type 2 Diabetes (GRADE) Study: A Comparative Effectiveness Randomized Clinical Trial. 2024 Apr 1; 47(4)589-593. Doi: 10.2337/dc23-1356. Epub ahead of print. PMID: 38252886.











